The recent announcement by Tesla of Powerwall, its new lithium-ion (Li-ion) based residential battery storage system, has caused quite a stir. It even raises the possibility of going off-the-grid, relying upon solar panels to generate electricity, and storing it with their own battery and using it on demand.
Yet the lithium-ion technology used by Tesla isn’t the only one on offer. In fact, each of the various battery technologies has its own strengths and weaknesses, and some might even be superior to lithium-ion for home installations. Here is a quick survey of current battery technologies, and some that are in development.
Battery power
All rechargeable batteries consist of two electrodes separated by an electrolyte (see diagram below). Two different reversible chemical reactions occur at the two electrodes. While charging, an “active species” – i.e. a charged molecule, such as lithium ions for Li-ion batteries – is stored in the anode. During discharge this migrates to the cathode. The chemical reaction occurs at a potential which can be used to power an external circuit.
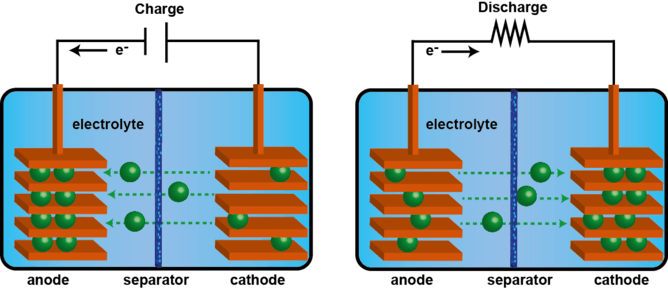
Each type of battery technology can be judged on a number of criteria, such as:
- Recyclability, which is the number of times it can be charged and discharged
- Energy density, which is a measure of the energy stored per unit mass, measured in Watt-hours (a measure representing a Watt of power output over an hour) per kilogram (Wh/kg)
- Specific density, which is the energy stored per unit volume, measured in Watt-hours per litre (Wh/l).
Which technology is best suited for a particular application depends on the demands of that role.
Lead-acid
The original rechargeable battery consists of concentrated sulphuric acid as the electrolyte (H₂SO₄), and lead (Pb) and lead dioxide (PbO₂) on both the anode and cathode, which are both converted to lead sulphate during charge and discharge.
Lead-acid batteries are still used in automobiles, caravans and in some electric relay grids. They have very high recyclability, thus a long lifetime. This is helped by short duration use and constant charging – i.e. always keeping the battery at nearly 100% charge – such as occurs in an automobiles. Conversely, slow charge and discharge significantly reduces lead-acid battery lifetime.
Although lead is toxic and sulphuric acid is corrosive, the battery is very robust and rarely presents a hazard to the user. However, if used in a residential installation, the greater size and volume of materials required will also increase the hazards.
The Li-ion Tesla Powerwall comes in 7 kilowatt-hours (kWh) or 10kWh versions. For the sake of comparison, we’ll look at what size battery would be required to power a four-person household that consumes 20kWh per day, which is approximately the national average for such homes.
Lead-acid batteries have energy density of 30 to 40Wh/kg and 60 to 70Wh/l. This means a 20kWh system will weigh 450 to 600kg and take up 0.28 to 0.33 cubic metres of space (not including the size or weight of the cell casing and other equipment). This volume is manageable for most households – it would roughly fit in a box 1 x 1 x 0.3 metres – but the weight will mean that it must be stationary.
Lithium-ion
The current premier rechargeable battery is based upon the movement of lithium (Li) ions between a porous carbon anode and a Li-metal oxide cathode. The composition of the cathode has a large effect on the performance and stability of the battery.
Currently lithium-cobalt-oxide exhibits superior charge capacity. However, it is more susceptible to breakdown than alternatives, such as lithium-titante or lithium-iron-phosphate, although these have lower charge capacity.
One common cause of faults is swelling of the cathode as Li ions are inserted within its structure along with the plating of the anode with lithium metal, which can become explosive. The chance of a breakdown can be reduced by limiting the charge/discharge rate, but instances of laptop or phone batteries exploding/catching fire are not uncommon.
The lifetime of the battery also depends heavily on the anode, cathode and electrolyte composition. Generally, the lifetimes of Li-ion are superior to lead-acid batteries, with Tesla reporting a lifetime of 15 years (5,000 cycles, at one cycle per day) for its 10 kWh Powerwall, based on a lithium-manganese-cobalt electrode.
The 10kWh Tesla Powerwall weighs 100kg and has dimensions of 1.3 x 0.86 x 0.18 metres. So for an average four-person household will require two units connected in series, coming to a total weight of 200kg and 1.3 x 1.72 x 0.18 metres or 0.4 cubic metres, which is lighter than lead-acid, but takes up more space.
These values equate to 100Wh/kg and 50Wh/l, which are lower than that reported for Li-cobalt oxide batteries (150-250Wh/kg and 250-360Wh/l), but in the range associated with the safer and longer lifetime Li-titanate (90Wh/kg) and Li-iron phosphate (80 to 120Wh/kg).
For the full story: https://theconversation.com/tomorrows-battery-technologies-that-could-power-your-home-41614?


516 Comments